16+ Chapter 9 Review Stoichiometry Section 2
The decomposition reaction exhibits first-order behavior at a quartz SiO 2 surface as suggested by the exponentially decaying plot of. 71 72 73 What is the molar mass of dipyrithione.

Pdf Cellulosic Substrates For Removal Of Pollutants From Aqueous Systems A Review 1 Metals Liliana Cerasella Indolean Academia Edu
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.

. 171 Review of Redox Chemistry. Types of chemical reactions. ASCII characters only characters found on a standard US keyboard.
6 to 30 characters long. However I find quite a few space errors in the text especially on page 141 chapter summary section of Chapter-2. The content and the information provided in the book are accurate.
It is a common element in the universe estimated at seventh in total abundance in the Milky Way and the Solar SystemAt standard temperature and pressure two atoms of the element bond to. A dandruff shampoo contains dipyrithione C10H8N2O2S2C10H8N2O2S2 which acts as an antibacterial and antifungal agent. How many moles of dipyrithione contain 82 102482 1024 atoms of N.
How many moles of dipyrithione are in 250 g. Nitrogen is the chemical element with the symbol N and atomic number 7. Enzymes are proteins that speed up chemical reactions necessary for life in which substrate molecules are converted into products.
Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table often called the pnictogens. K 297 x 10-19 s 2 m 3 T 2R 3 Newton was able to combine the law of universal gravitation with circular motion principles to show that if the force of gravity provides the centripetal force for the planets nearly circular orbits then a value of 297 x 10. B While the equipment used by Alma Levant Hayden in this 1952 picture might not seem as sleek as you might find in a lab today her approach was highly methodical and carefully.
Modification of work. This represents a 32 or 151 ratio of hydrogen to chlorine present for reaction which is greater than the stoichiometric ratio of 11. 171 Review of Redox Chemistry.
Lets try and see if n 5 and g 2 follows the general chemical formula of cyclic. 107 In brief distortions of this molecule can lead to several geometries that are described by the same Coulomb matrix. When 1 mole of NH 4 2 Cr 2 O 7 is dissolved it results in 3 moles of ions 1 mol of Cr 2 O 7 2 anions and 2 mol of NH 4 cations within the solution Figure 811.
Calculating Free Energy Change for a Coupled Reaction. Figure 12 a This portrayal shows an alchemists workshop circa 1580. A plot of A versus t for a zero-order reaction is a straight line with a slope of k and a y-intercept of A 0Figure 1211 shows a plot of NH 3 versus t for the thermal decomposition of ammonia at the surface of two different heated solids.
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Join an activity with your class and find or create your own quizzes and flashcards. Molecular and Ionic Compound Structure and Properties.
Must contain at least 4 different symbols. Modification of work by the Italian voiceFlickr. 174 Potential Free Energy.
174 Potential Free Energy. 4 Work energy and force. For example Figures 23 and 24 would be more appropriate when electron and molecular geometries are introduced in Chapter 9 and the titration curves that appear in conceptual problem 3 section 49 would fit better after acidbase equilibria buffers and titrations are formally presented in Chapter 16 Consistency rating.
There are 5 carbon atoms so n 5 and there are two rings so g 2. Modification of work by vxlaFlickr. Likewise the Na and Cl atoms in NaCl have an electronegativity difference of 21 and the Mn and I atoms in MnI 2 have a difference of 10 yet both of these substances form ionic compounds.
79 of exam score. Hydrogen therefore is present in excess and chlorine is the limiting reactant. How many moles of C are in 250 g of dipyrithione.
The reason for this property of SiC fibers is that most of them contain additional elements like oxygen titanium andor aluminum yielding a tensile strength. United Nations Sustainable Development Goals - Time for Global Action for People and Planet. The non-uniqueness can be demonstrated using as an example acetylene C 2 H 2.
As was previously demonstrated in this chapters section on entropy the spontaneity of a process may depend upon the temperature of the system. They can also be amorphous or have inhomogeneous chemical composition which develops upon pyrolysis of organic precursors. State the periodic law and explain the organization of elements in the.
6 Acid-base chemistry Ÿ P Ch. Ÿ 3-4 CARS practice passages Day 15 Day 16 Day 17 Day 18 Day 19 Day 20 Day 21 Ÿ FULL-LENGTH review Ÿ Study CP MQL for. By the end of this section you will be able to.
3 Molecules and stoichiometry Ÿ GC Ch. The Ideal Gas Law 93 Stoichiometry of Gaseous Substances Mixtures and Reactions 94 Effusion and Diffusion of Gases. The formula of spiro22pentane is C5H8.
For example imagine combining 3 moles of H 2 and 2 moles of Cl 2. Check Your Learning The K sp of PbI 2 is 14 10 8. To discuss the relationship between the concentration of a solution and the resulting number of.
However although the last digit 5 is not. When performing calculations stepwise as in Example 317 it is important to refrain from rounding any intermediate calculation results which can lead to rounding errors in the final resultIn Example 317 the molar amount of NaCl computed in the first step 1325 mol would be properly rounded to 132 mol if it were to be reported. Although alchemy made some useful contributions to how to manipulate matter it was not scientific by modern standards.
173 Electrode and Cell Potentials. Besides answers of the exercise on the page 207 problems- 5 7 9 11 does not match with questions. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site a specialized area on the enzyme that accelerates the most difficult.
92 Relating Pressure Volume Amount and Temperature. Calculate the molar solubility of leadII iodide. The dissolution stoichiometry shows a 11 relation between moles of calcium ion in solution and moles of compound dissolved and so the molar solubility of CaOH 2 is 69 10 3 M.
The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Ÿ Study 1 weak chapter Ÿ AAMC Section Bank review Ÿ Study BB MQL for 1 hr Ÿ 3-4 CARS practice passages. 173 Electrode and Cell Potentials.
Ceramic fibers in CMCs can have a polycrystalline structure as in conventional ceramics. Once you join your AP class section online youll be able to access AP Daily videos any assignments from your teacher and your assignment results in AP Classroom.

Ppt Modern Chemistry Chapter 9 Stoichiometry Powerpoint Presentation Id 2956403

One Guest Or Two A Crystallographic And Solution Study Of Guest Binding In A Cubic Coordination Cage Taylor 2020 Chemistry 8211 A European Journal Wiley Online Library

Diastereoselective Control Of Tetraphenylethene Reactivity By Metal Template Self Assembly Kennedy 2019 Chemistry A European Journal Wiley Online Library
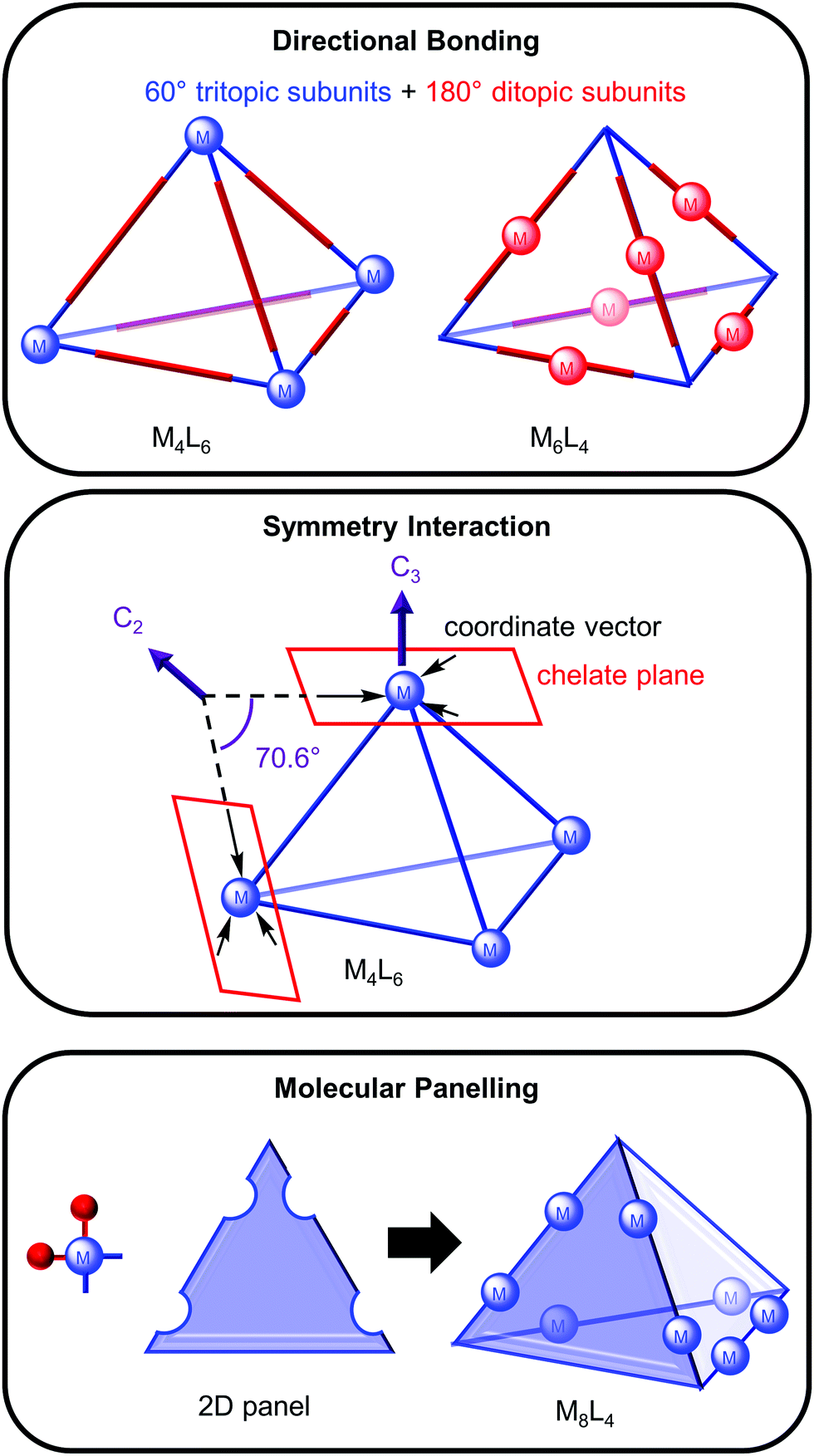
Metallosupramolecular Cages From Design Principles And Characterisation Techniques To Applications Chemical Society Reviews Rsc Publishing Doi 10 1039 D1cs01143j

Chapter 9 Answer Key Practice Questions Only Quia

Chapter 9 Review

Chapter 9 Stoichiometry Chapter Review Answers

09 Answer Key Pdf

Ch 9 Quizzes Sections 1 2 3 Answer Keys Pdf Section Quiz Introduction To Stoichiometry In The Space Provided Write The Letter Of The Term Or Phrase Course Hero

The Equilibrium Constant Kc Part 2 Teaching Resources

Ppt Modern Chemistry Chapter 9 Stoichiometry Powerpoint Presentation Id 2956403

Focus Tg4 Kssm Chemistry Terbitan Penerbitan Pelangi Sdn Bhd Flip Ebook Pages 1 48 Anyflip

Ppt Modern Chemistry Chapter 9 Stoichiometry Powerpoint Presentation Id 2956403

Ppt Modern Chemistry Chapter 9 Stoichiometry Powerpoint Presentation Id 2956403

Chapter 9 Practice Test Pdf Name M Class Date Ain Chapter Test 3 Chapter Stoichiometry Part I In The Space Provide Write The Ietter Oi The Course Hero
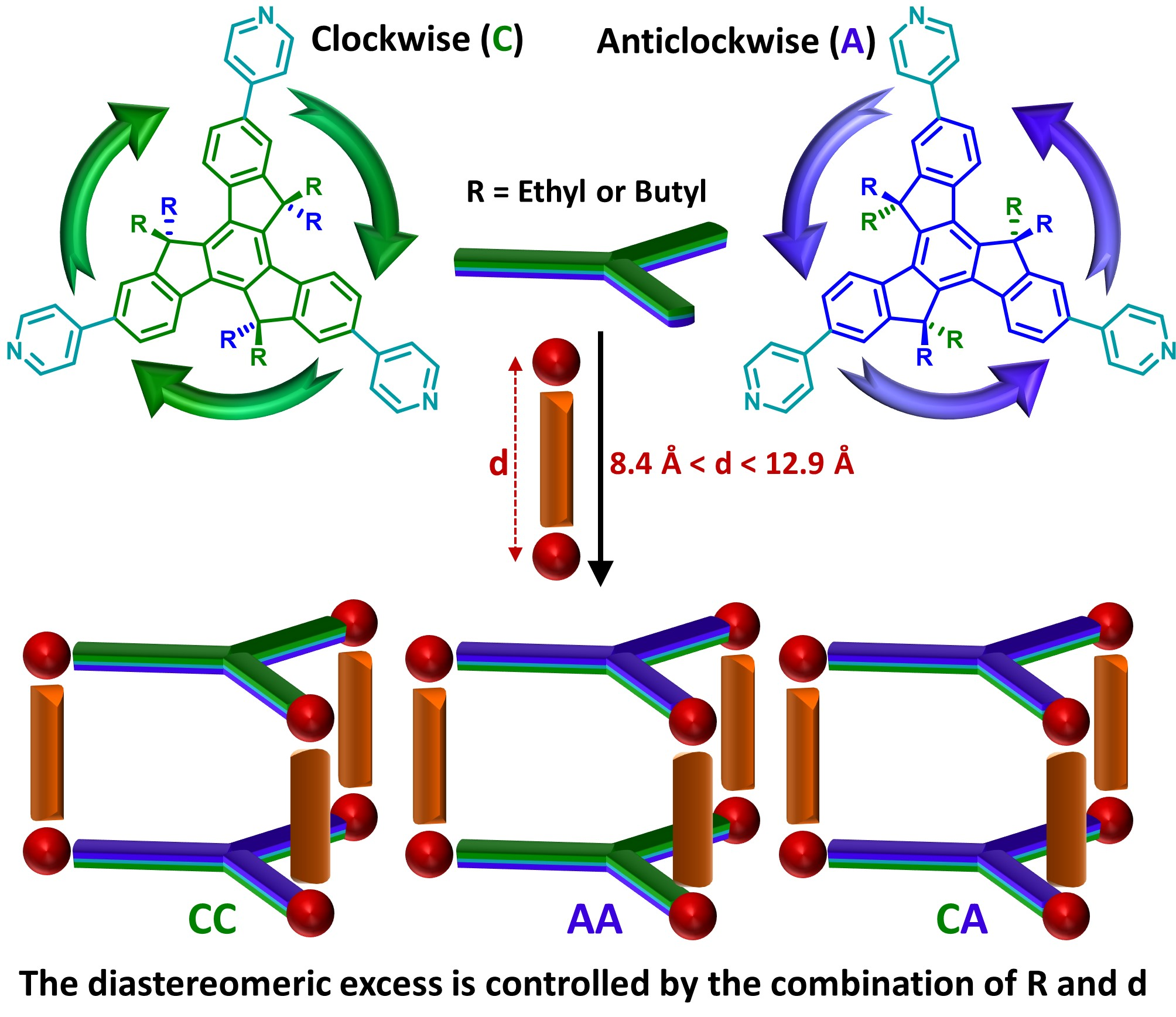
Inorganics Free Full Text Controlling Chiral Self Sorting In Truxene Based Self Assembled Cages

Modeling Of Transport Phenomena In Fixed Bed Reactors For The Fischer Tropsch Reaction A Brief Literature Review